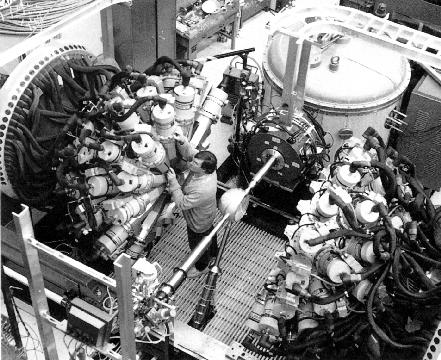
Argonne National Laboratory
A $20 million national traveling physics instrument, Gammasphere was built to study the complex structure and behavior of nuclei by fusing lighter nuclei into heavier ones and observing gamma rays - a form of extremely high-energy light - emitted when the new nuclei's component protons and neutrons settle into stable configurations.
A consortium of scientists from the national laboratories and many universities designed and built Gammasphere. The project was coordinated by scientists at Lawrence Berkeley National Laboratory, and the device first assembled there. The detector array was moved to the Physics Division at Argonne in the fall of 1997.
The detector allows physicists to explore regions of the nuclear landscape only glimpsed in previous research: "the proton dripline," where highly unstable nuclei filled to overflowing with protons - reside for fractions of a second before shattering or reverting to more stable forms. The research is giving physicists their first experimental looks at the astronomical process by which some of the elements in the universe are formed.
 Argonne National Laboratory |
|
| See also the
Table of Nuclides.
|
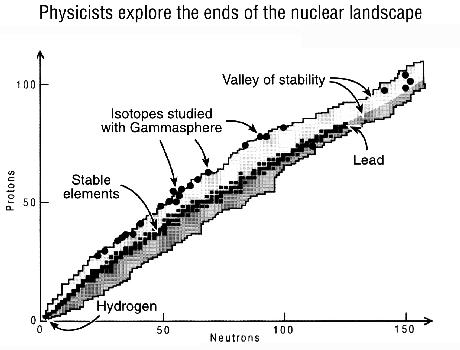
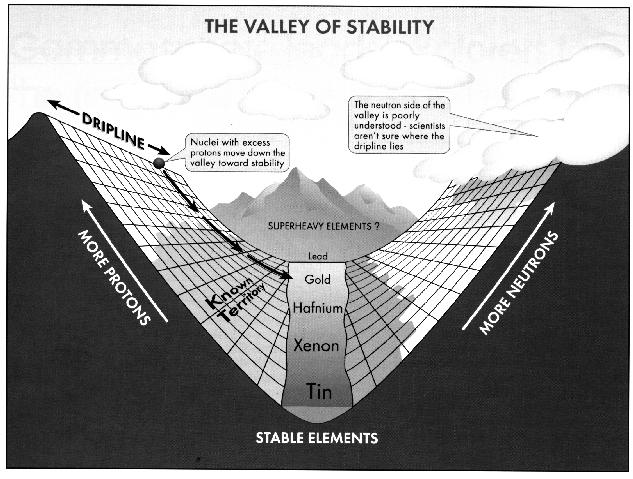
Where and why the valley ends are not known. Scientists presume that at some point, protons are overwhelmed by Coulomb repulsion (the electrostatic force that makes alike objects, such as the positively charged protons in the nucleus, repel each other). Such huge nuclei would spontaneously undergo fission (break apart). However, up to element 112 and probably beyond, the intricate mechanics of the attractive nuclear force keeps Coulomb repulsion in check.
Lining the valley on either side of the stable elements are isotopes, variations on each element with different numbers of neutrons. For example, a hydrogen atom with an extra neutron is deuterium, which would be plotted just to the right of hydrogen, slightly uphill on the neutron side of the valley.
Physicists wish to explore along, or even past, the rim of the valley of stability where nuclei survive for only a short time. "Physicists have always been fascinated with the ends of the nuclear landscape," said Argonne Physicist Kim Lister, who coordinated the installation of Gammasphere.
Fusing nuclei isn't easy. Coulomb repulsion creates a barrier between nuclei that can be overcome only with speed.
Stars, novae and supernovae accelerate nuclei to fusion speeds with million- or billion-degree temperatures. Argonne physicists use the Argonne Tandem Linear Accelerator System, or ATLAS.
ATLAS accelerates ions (atoms with one or more electrons removed) to between 10 and 15 percent of the speed of light. Then they are smashed head-on into thin-film targets.
Only one in 100,000 of the particles pouring down the beam pipe each second finds itself on a direct collision course with a nucleus in the target. The nuclei coalesce, and that's when things get interesting.
The newly created nucleus is a large, unstable conglomeration of protons and neutrons. It may be high on the side of the valley of stability, and it wants to get back down to a more stable configuration. The nucleus also may be vibrating like a rung bell or, if the collision was off-center, spinning rapidly and deformed into the shape of a football or pancake. The new nucleus is also extremely hot.
Hot matter has two favorite ways to cool off: boil and radiate.
In a hot drop of water, for example, the hottest molecules - those moving fastest - will escape the droplet as steam, taking with them some of the droplet's total energy. The remaining molecules average energy decreases, and the droplet cools down. The drop will also radiate energy as infrared photons, a form of light we feel as heat, as excited electrons in the hydrogen and oxygen atoms drop from higher energy levels to lower ones.
A hot atomic nucleus cools off in similar ways. It boils off protons and neutrons, cooling itself by evaporation. Eventually, in less than an attosecond (a millionth of a millionth of a millionth of a second) or so, the nucleus loses enough protons and neutrons to find a relatively stable configuration. As the protons and neutrons settle into their new energy levels, they radiate high-energy photons called gamma rays. Finally, on a timescale of seconds to years, the nuclei will trace a relatively well-known track down the hillside to stability.
The rules governing these reactions are those of quantum mechanics and quantum mechanics is a science of probability. Like that one-in-a-million chance that a flipped coin will land on its edge, occasionally a nucleus won't take the path of least resistance. It will hold onto the extra nucleons, at least for a little while, and meander along the valley rim and perhaps even find a temporary resting place there.
Gammasphere is being used to study these rare events. The characteristic pattern of gamma rays emitted as the new nuclei settle into novel configurations is rare, and buried in an avalanche of gamma rays from other nuclei fleeing down the hillside, but Gammasphere can detect the signal with ease.
When struck by gamma rays, the germanium crystals emit pulses of electricity. The sequence of pulses from the 100 detectors surrounding the target provides clues to what's going on as nuclei fuse and cool.
Historically, physicists used a single such germanium detector, or a few, and moved the detector(s) around the target over a period of days to gather a full picture of gamma-ray emissions.
After cooling by evaporation and gamma-ray emission, the nuclei fly on for another 30 feet through another detector a spectrometer called the Fragment Mass Analyzer (FMA). The FMA measures their mass and records their eventual decay. Correlating the two events - prompt gamma-ray emission and eventual decay - enables physicists to pick out one interesting nucleus from a background of 10 million less interesting ones.
This study, in addition to other recent experiments at Argonne and other institutions, has confirmed theories of why some superheavy nuclei can remain relatively "stable" - for a few thousandths of a second - when by all classical expectation they should spontaneously fly apart.
The stability, Lister explained, is due in part to their elongated shapes. Unlike lighter elements, nobelium is born elongated by about 20 percent, like a football. Physicists were able to determine the shape because the nobelium nuclei were spinning. "A deformed nucleus makes a good antenna," Lister said. "As they spin, they radiate a characteristic pattern of gamma rays, from which their shapes can be inferred."
The elongated shape allows particularly strongly bound quantum configurations, and decreases the average Coulomb force acting on the protons.
The surprise was that nobelium holds together despite high rates of spin that should be enough to tear it apart.
"It appears the nobelium nucleus is very stable against rotation," Lister said. "There's no reason to believe we cant do this sort of experiment for very much heavier elements."
 Argonne National Laboratory |
|
| Physicist Ernst Rehm leads a group studying "explosive
nucleosynthesis," the process by which heavy elements are formed in
supernovae and novae. |
Rehms group created tiny amounts of nickel-56 in Argonne's Intense Pulsed Neutron Source and transported the material about a mile across the laboratory to ATLAS.
Because of technical limitations, they could coax only one million ions per second from the source (of which about 10,000 made it through the acceleration process) "a million times less than we're used to," Lister said. But Gammasphere clearly picked up the signal of nickel-56 nuclei fusing with those in a molybdenum target, forming isotopes of dysprosium.
"If the beams were 100 times stronger, we could do really cutting-edge stuff," Lister said.
To progress much farther in the studies of these fundamental processes will require intense beams of exotic nuclei. Argonne has proposed using ATLAS as a base for an "Exotic Beam Facility." The facility would provide a shortcut right to the heaviest nuclei, and nuclei at the proton dripline, up to the heaviest masses.
Much of physicists' understanding of both nuclear structure and synthesis is still based on theory developed to explain the properties of stable and near-stable nuclei. Many questions are open concerning how the interactions between nucleons generate the structures and binding energies found in nature.
One particularly challenging question involves nuclei near the limits of stability, where the least-bound nucleons can actually appear outside the nucleus. These loosely bound nuclei form a strange, intermediate state between unconfined nucleons and those tightly bound in normal nuclear matter.
To study loosely bound nuclei, especially on the poorly explored, neutron-rich side of the valley of stability, requires beams of radioactive isotopes such as those that would be supplied by the Exotic Beam Facility.
In the meantime, Argonne physicists and their colleagues from many other laboratories in many nations continue to explore the new frontier with the tools available today. "By banding larger groups of researchers to pool beam time," Lister said, "data- gathering times of weeks instead of days will allow scientists to examine more subtle processes and explore regions even farther from stability."
It's the equivalent of keeping the camera shutter open longer to take a
more detailed photograph of space. In this case, however, the tiny
flashes of light are telling scientists not how big the universe is,
but how it works.